MAINZ/SHANGHAI, September 6, 2025 – BioNTech SE and Duality Biologics have announced a significant milestone in their oncology pipeline with the Phase 3 success of trastuzumab pamirtecan (BNT323/DB-1303), an investigational next-generation antibody-drug conjugate (ADC) targeting HER2-positive metastatic or unresectable breast cancer.
The pivotal China-based trial (NCT06265428) met its primary endpoint of progression-free survival (PFS) in patients who had previously received trastuzumab and taxane-based chemotherapy. Interim results, reviewed by the Independent Data Monitoring Committee and evaluated by Blinded Independent Central Review, position the candidate as a potential new treatment option for patients facing limited choices.
Breakthrough in the BioNTech–DualityBio Collaboration
This development marks the first late-stage success from BioNTech’s oncology pipeline and from its strategic collaboration with DualityBio, launched in April 2023 to accelerate ADC therapies for solid tumours.
“This is a milestone in the fruitful collaboration with our colleagues at DualityBio,” said Prof. Özlem Türeci, M.D., Chief Medical Officer and Co-Founder at BioNTech. “We believe that trastuzumab pamirtecan is an ADC candidate with enormous potential which makes it an important asset in our global oncology strategy including combinational approaches. It is the first of our late-stage oncology programs to meet its primary endpoint in a pivotal Phase 3 trial.”
DualityBio’s Global Medical Officer Dr. Hua Mu emphasised the clinical relevance: “The positive Phase 3 data and trastuzumab pamirtecan meeting the primary endpoint at the interim analysis indicate the potential of the BNT323/DB-1303 program to become a new treatment option for breast cancer patients. Together with our partner BioNTech, we plan the development of trastuzumab pamirtecan in further tumour types towards BLA application in other regions including the United States and the European Union.”
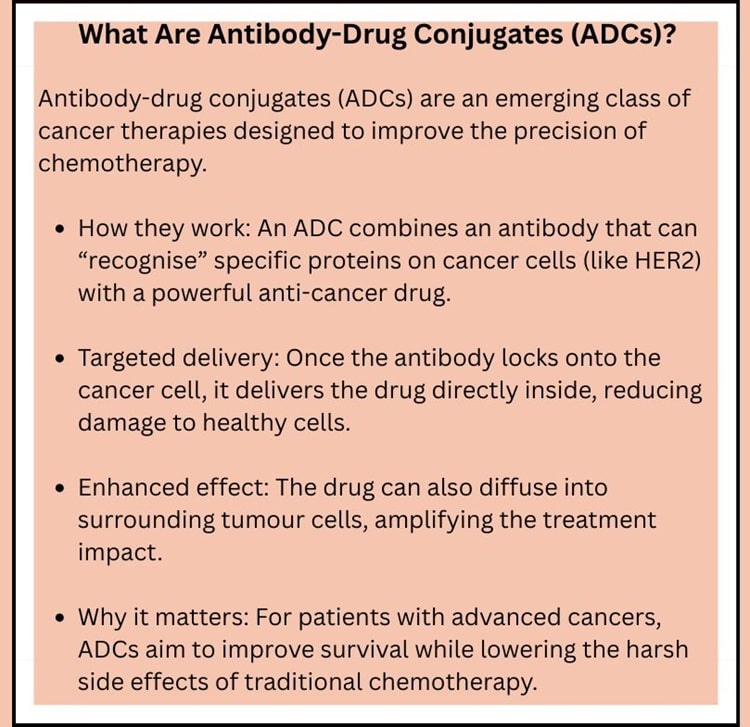
Understanding the Candidate: A Targeted Approach
BNT323/DB-1303 belongs to a class of drugs known as antibody-drug conjugates, which combine the precision of antibodies with the potent cancer-killing effect of chemotherapy. Unlike conventional chemotherapy — which risks damaging healthy cells — ADCs are engineered to selectively deliver cytotoxic agents directly into tumour cells
Specifically, trastuzumab pamirtecan targets HER2, a protein present on the surface of many breast cancer cells and implicated in aggressive tumour growth. Upon binding to HER2, the ADC delivers its chemotherapeutic payload inside the cancer cell, triggering destruction while potentially limiting systemic side effects.
Addressing an Urgent Medical Need
Breast cancer remains the most commonly diagnosed cancer globally and the leading cause of cancer-related deaths among women. In China alone, there are over 350,000 new cases annually, making it the second most common malignancy among Chinese women. Importantly, more than half of breast cancers express HER2, underscoring the critical role of targeted therapies.
While early-stage breast cancer can be curable in 70–80% of cases, advanced stages have limited treatment options and no curative therapies. The success of BNT323/DB-1303 offers new hope for patients living with HER2-positive disease, particularly those with unresectable or metastatic conditions.
Expanding Global Clinical Development
Beyond China, the global Phase 3 DYNASTY-Breast02 trial (NCT06018337) is underway to evaluate trastuzumab pamirtecan in HR-positive, HER2-low metastatic breast cancer. This programme builds on positive Phase 1/2 safety and efficacy data in HER2-expressing solid tumours.
Under the commercial agreement, BioNTech holds global rights outside Mainland China, Hong Kong, and Macau, while DualityBio retains rights in those regions. Both companies see this candidate as a cornerstone in their oncology pipelines, with ambitions to expand development to additional tumour types, including endometrial, colorectal, lung, and oesophageal cancers.
The successful interim readout positions trastuzumab pamirtecan as a strong contender in the competitive HER2 oncology space, currently led by approved ADCs like trastuzumab emtansine (T-DM1). With regulatory discussions in China imminent and global trials progressing, the programme could reshape treatment pathways for breast cancer and beyond.
For patients facing advanced HER2-positive disease, the data represents more than scientific progress — it offers the promise of renewed hope.

